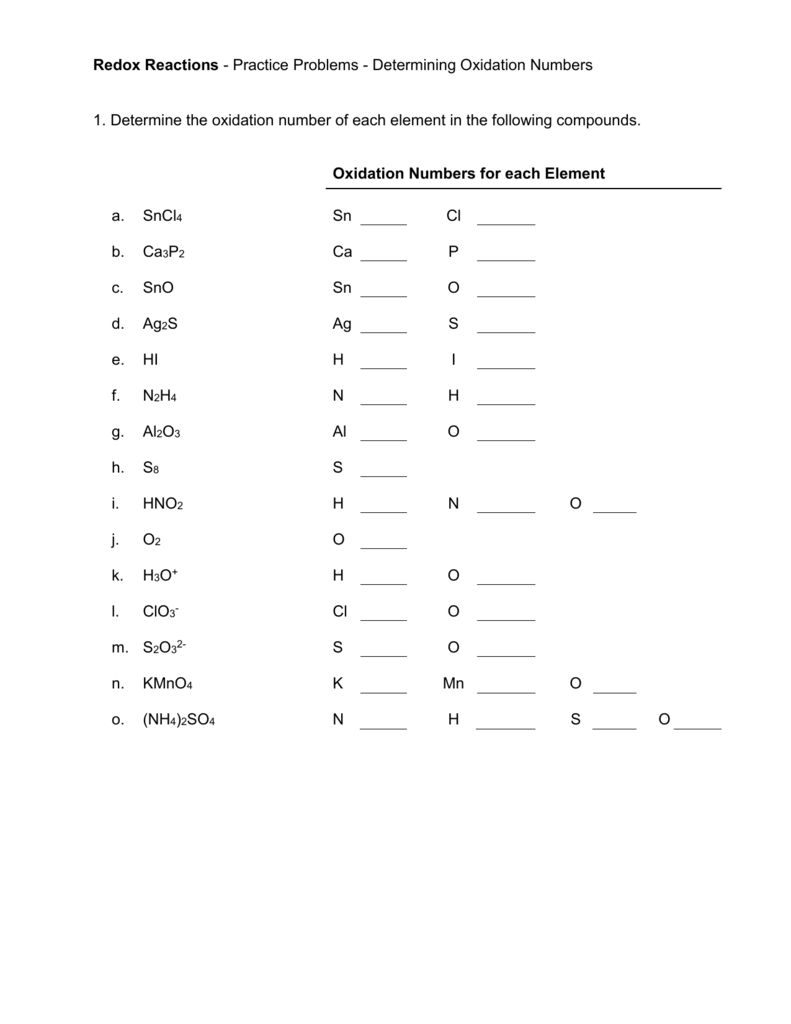
Oxidation State Worksheets
Give the oxidation number of each kind of atom or ion. sulfate b. Sn c. S2- d. Fe3+ e. Sn4+ f. nitrate g. ammonium Calculate the oxidation number of chromium in each of the following. Cr2O3 b. Na2Cr2O7 c. CrSO4 d. chromate e. dichromate

why do transition metals have multiple oxidation states
Oxidation numbers Student worksheet: CDROM index 30SW Discussion of answers: CDROM index 30DA Topics Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of assigning oxidation numbers - electronegativity values and oxidation number rules. Level Very able post-16 students.

Worksheet Oxidation Numbers Answer Key
The oxidation number of oxygen in most compounds is \(-2\). The oxidation number of hydrogen in most compounds is \(+1\). The oxidation number of fluorine in all compounds is \(-1\). Other halogens usually have an oxidation number of \(-1\) in binary compounds, but can have variable oxidation numbers depending on the bonding environment.

Worksheet Assigning Oxidation Numbers Key.doc
The Assigning Oxidation Numbers to Elements Worksheet consists of two pages:Page 1: - Students will write the SIX (6) Rules for determining the Oxidation Number to Monotamic (Unreacted) Elements, Elements in a Compound, and Elements in a Polyatomic Ion.- THREE (3) Practice Problems in which students must determine the Oxidation State of.

ASSIGNING OXIDATION NUMBERS WORKSHEET
Reduction 1⁄2 Reaction: 0 Br2 + 2e ̄ à 2Br ̄. Now, combine the new half-reactions into a final equation. Note that all of the electrons have cancelled out: New Oxidation 1⁄2 Reaction: 2K0 à 2K+ + 2e ̄. Reduction 1⁄2 Reaction: + Br2 0 + 2e ̄ à 2Br ̄. Balanced Ionic Equation: 2K0 + Br2.

assign oxidation numbers
2. The oxidation number of a monatomic ion equals the charge on the ion. 3. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in a compound is always -1. 5. Oxygen has an oxidation number of -2 unless it is combined with F (when.
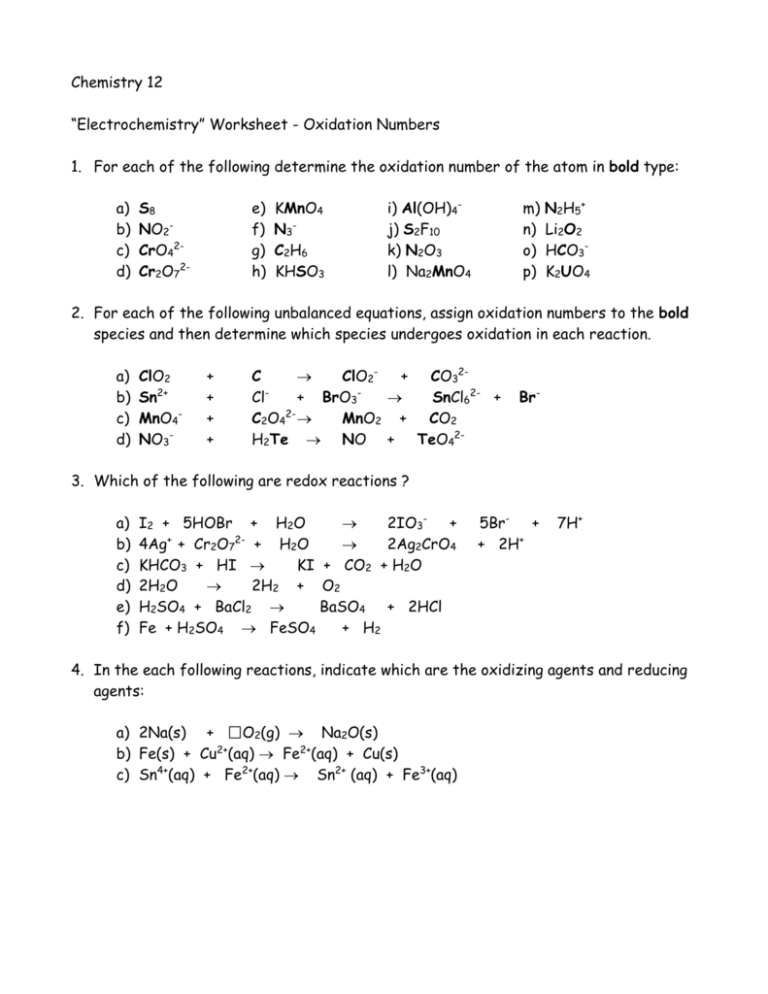
Worksheet 2. Oxidation Numbers Worksheet 2
One way of reflecting this is through changes in assigned oxidation numbers. Oxidation numbers are real or hypothetical charges on atoms, assigned by the following rules: Atoms in elements are assigned 0. All simple monatomic ions have oxidation numbers equal to their charges. (e.g., all Group 1 ions are +1; all group 2 ions are +2; all the.

Oxidation Numbers Worksheet With Answers
Worksheets: General Chemistry (Traditional)

️Oxidation Numbers Worksheet And Answers Free Download Goodimg.co
By assigning oxidation numbers to the atoms of each element in a redox equation, we can determine which element is oxidized and which element is reduced during the reaction. In this video, we'll use this method to identify the oxidized and reduced elements in the reaction that occurs between I⁻ and MnO₄⁻ in basic solution. Created by Sal.
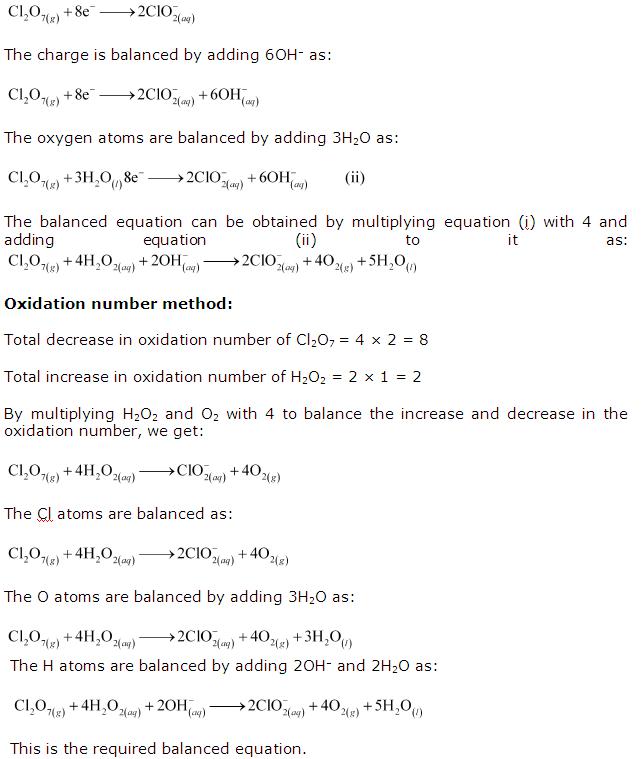
Oxidation Number Worksheet Answer Key
Oxidation numbers are assigned to elements using these rules: Rule 1: The oxidation number of an element in its free (uncombined) state is zero — for example, Al(s) or Zn(s). This is also true for elements found in nature as diatomic (two-atom) elements: H2, O2, S8.
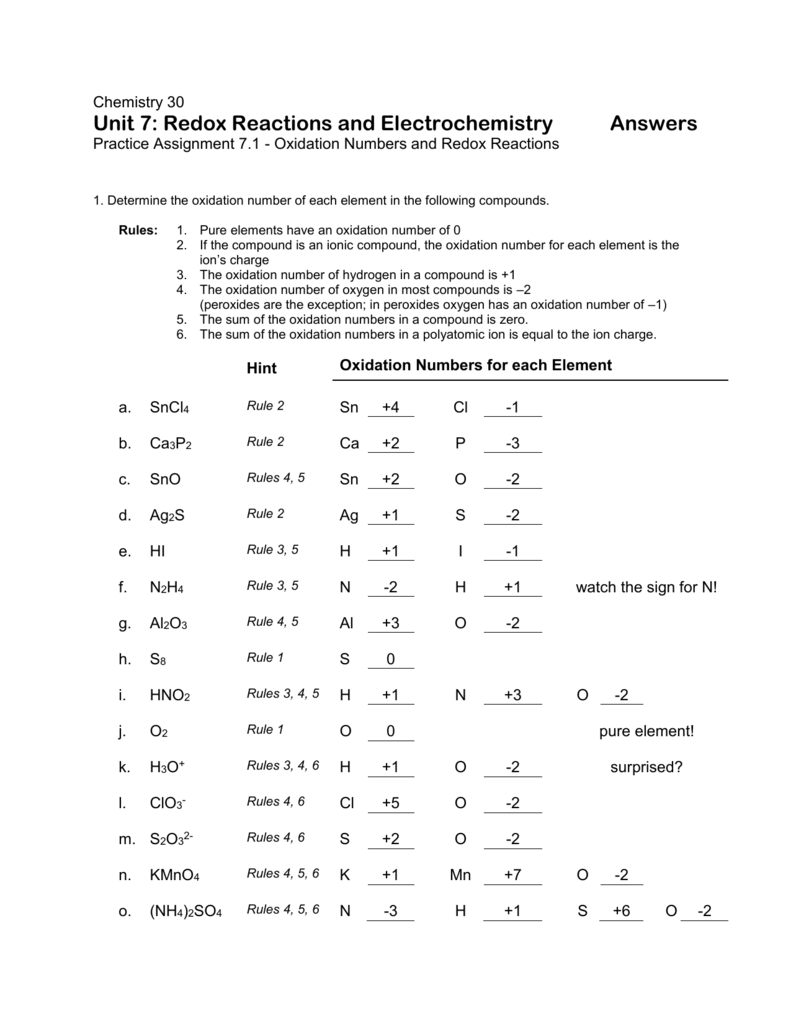
5.1 Oxidation Numbers
The oxidation number of any uncombined element is 0. The oxidation number of a monatomic ion equals the charge on the ion. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. The oxidation number of fluorine in a compound is always -1
Charting Oxidation Number Worksheet Answer Key Worksheets Joy
ASSIGNING OXIDATION NUMBERS WORKSHEET Part A: In the following questions, give the oxidation number of the indicated atoms/ion. N in N2O3 __________ S in H2SO4 __________ C __________ C in CO __________ Na in NaCl __________ H in H2O __________ Ba in BaCl2 __________ N in NO2 - __________ S in Al2S3 __________ S in HSO4 - __________
Assigning Oxidation Numbers Worksheet Answer Key Escolagersonalvesgui
The number of valence electrons on an atom is equal to its group number. In a cation, the oxidation number is equal to the number of these electrons which have been removed. Transition metal cations have a configuration dz where Z is the number of valence electrons left over after ionization: Z = number of valence electrons on atom- charge of.

Aluminum Aluminum Oxidation Number
Z = number of valence electrons on atom- charge of cation (1) (1) Z = number of valence electrons on atom - charge of cation. = group number- oxidation number (2) (2) = group number - oxidation number. For example: Ni is in group 10 so Ni2+ N i 2 + has (10 - 2) = 8 valence electrons left: it has a d8 d 8 configuration.

Using Oxidation numbers to find formulas (polyatomic ions) worksheet
a. The charge on all free elements is zero. b. The charge on all metals of group 1 of the periodic table is +1 c. The charge on all metals of group 2 of the periodic table is +2 d. The charge on aluminum is +3 e. The charge on hydrogen is +1, except in hydrides where it is -1 f.
16 Best Images of Organic Oxidation Reactions Worksheet Balancing
A monoatomic ion has an oxidation number equal to its charge. For example, the oxidation number of the oxygen in the oxide ion, O 2-, is -2. The sum of the oxidation numbers in a polyatomic ion is equal to the charge on the ion. Let's examine the oxidation numbers of some common elements. Notice the periodic trend among the main-group.